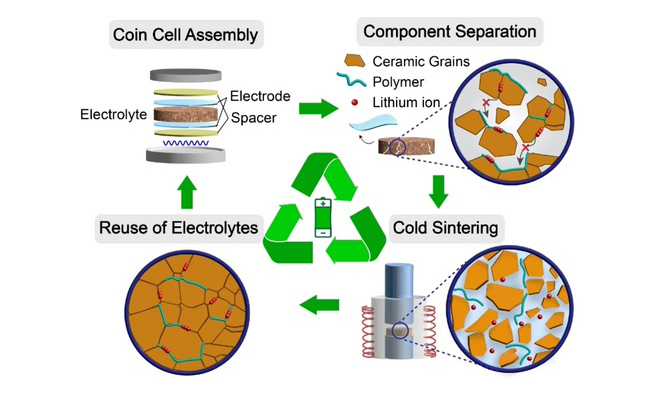
A team at Penn State has reported using cold sintering to reprocess solid-state composite electrolytes.
According to research published in an open-access paper in the journal ChemSusChem, the technique was used to reprocess Mg- and Sr-doped Li7La3Zr2O12 with polypropylene carbonate (PPC) and lithium perchlorate (LLZO–PPC–LiClO4).
The low sintering temperature allows co-sintering of ceramics, polymers, and lithium salts, leading to the re-densification of the composite structures. Reprocessed LLZO–PPC–LiClO4 exhibits densified microstructures with ionic conductivities exceeding 10−4 S/cm at room temperature after five recycling cycles. All-solid-state lithium batteries fabricated with reprocessed electrolytes exhibit a high discharge capacity of 168 mAhg−1 at 0.1 C, and retention of performance at 0.2 C for over 100 cycles.
“We directly reprocessed LLZO–PPC–LiClO4 composite electrolytes using cold sintering and demonstrate recyclability with modest loss in ionic conductivity. Cold sintering, a low-temperature sintering technology (<300° C), is a process that enables the densification of composites comprised of ceramics, polymers, and salts. Driven by pressure and heat, densification happens with the assistance of a transient liquid phase, which functions as a medium to aid in the dissolution and regulate the precipitation and nucleation processes,” the researchers wrote.
Source: Green Car Congress, ChemSusChem